Thursday, July 13th, 2023
Shepherd Chemical Explores a Greener Alternative for Converting Methane to Benzene

Written by Jacek Pecyna, R&D Chemist
In collaboration with Professor Sheima Khatib from Virginia Tech, Shepherd Chemical has been exploring the use of molybdenum-based catalysts on zeolite support for converting methane to benzene. This blog details the promising benefits this method may create, in terms of both sustainability and US economics.
Aromatic compounds are present in our daily life in different forms such as polymers, pharmaceuticals, solvents, packaging, and many others.
One of the most known representatives of this class of compounds is benzene. Predominantly, it is obtained via catalytic reforming and steam cracking of petroleum as well as coal processing. The shift towards production of lighter hydrocarbons in the US has led to underproduction of benzene. Currently, about 15% of domestic demand for benzene, carrying the value of nearly $1B/year, is imported to the US from other countries.
Given the economic and environmental impact of current methods of synthesis of benzene, Shepherd Chemical has decided to pursue a more feasible and greener alternative to the current production routes of benzene from abundant domestic shale gas. The positive impact of this method could be even higher if one envisions the use of methane lost due to flaring and leaking. More importantly, the proposed method would place the US in a position of benzene exporter and create an enduring economic effect.
In collaboration with Prof. Sheima Khatib from Virginia Tech, Shepherd has been exploring the use of molybdenum-based catalysts on zeolite support for converting methane to benzene.
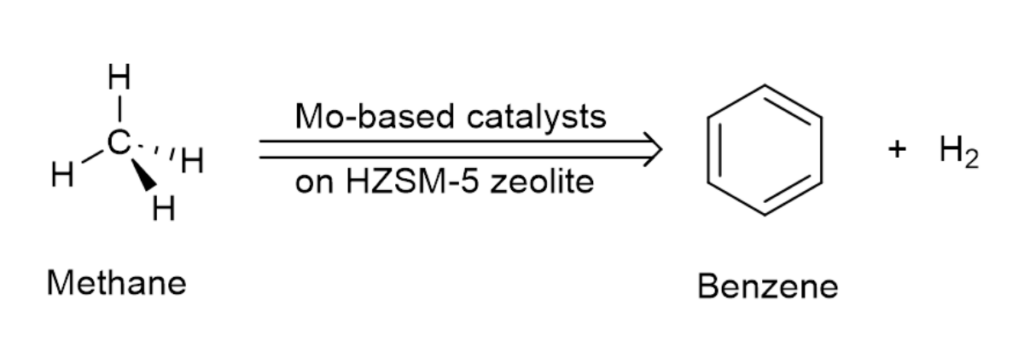
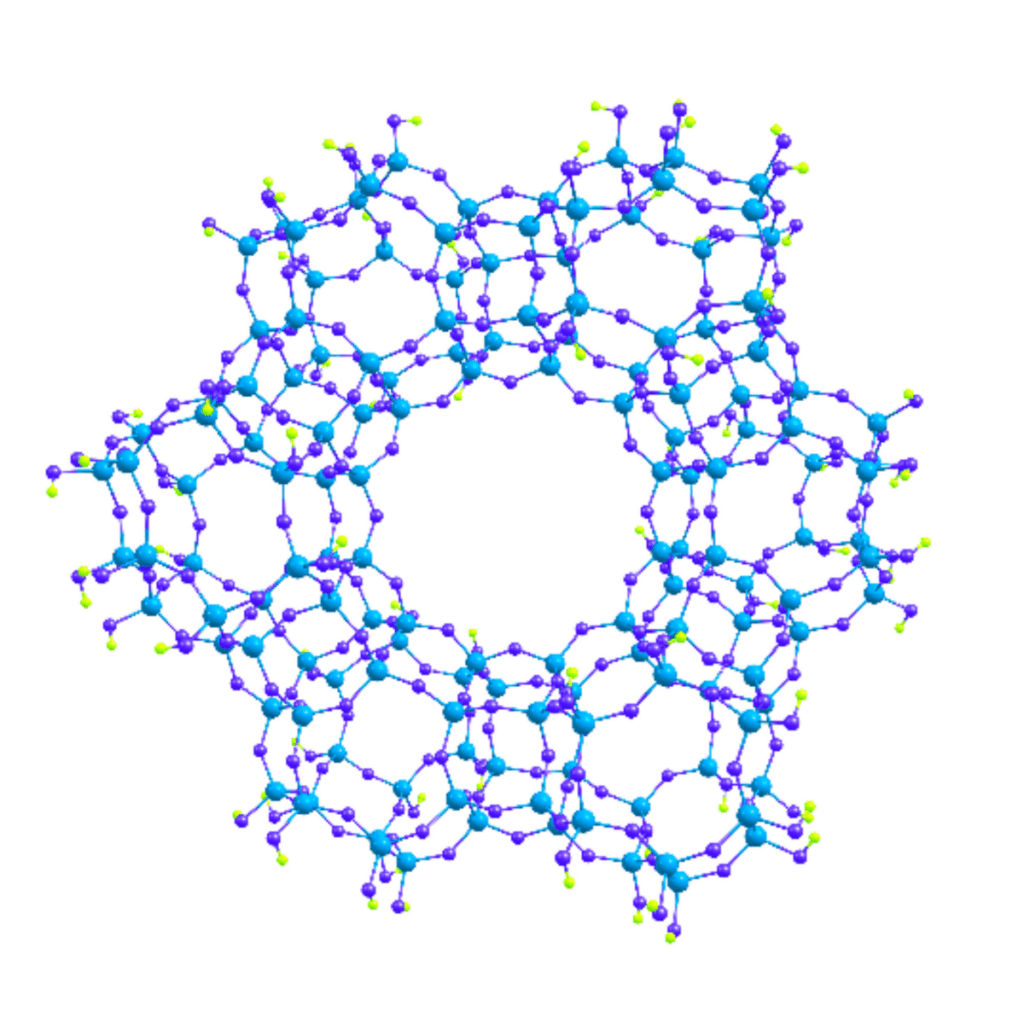 Zeolites have long been known to exhibit catalytic properties. In the current work, however, it is mostly the molybdenum species that catalyze the process of dehydroaromatization. Zeolite ZSM-5 serves mainly as the support, and we take advantage of its internal structure. Zeolites are a group of aluminosilicate minerals that form a three-dimensional framework of cavities and channels.
Zeolites have long been known to exhibit catalytic properties. In the current work, however, it is mostly the molybdenum species that catalyze the process of dehydroaromatization. Zeolite ZSM-5 serves mainly as the support, and we take advantage of its internal structure. Zeolites are a group of aluminosilicate minerals that form a three-dimensional framework of cavities and channels.
As it happens, the internal dimensions of the ZSM-5 channels can accommodate only molecules of benzene or smaller species. More complex aromatices are formed on the external surface of the catalyst support. In our work, we have developed a method that ensures that precursors to the Mo-based catalysts are trapped inside the zeolite channels rather than reside on its external surface. We also made sure that the precursors are evenly distributed over the support. That is important to enhance the catalyst’s activity and make it more resistant to deactivation.
The process of preparation of the pre-catalysts requires precise control of reaction conditions. For this purpose, we utilize simultaneous addition. This technique is regularly used in the chemical industry to produce a consistent product due to the fact that the reaction conditions are maintained consistent throughout the process. This way, we have successfully prepared pre-catalysts with different metals loadings which, upon further treatment, yielded benzene and other products in Prof. Khatib’s laboratory.
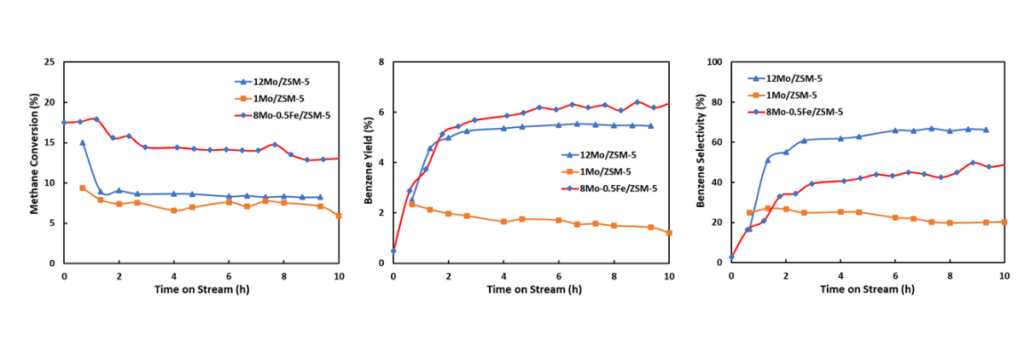
Some of the materials the Shepherd team has made contain two metals, molybdenum and iron. The purpose of the latter is to improve the catalyst’s stability and performance. Below, graphs showing methane conversion (left), benzene yield (right) and benzene selectivity (bottom) using three select catalysts prepared at Shepherd Chemical.
We look forward to providing updates on our work in this space! In the meantime, if you’d like to connect with Shepherd regarding any of the information presented above, please contact our Research & Development leader, Rob Hart, on LinkedIn. Alternatively, you can also contact us through our online form or by calling 513-731-1110.